There are different sorts of radiation: non-ionizing and ionizing radiation. Examples of non-ionizing radiation are: visible light and radio waves. Radioactive substances emit ionizing radiation. It is precisely due to this property that these substances are often used. However, it is this same property that makes radioactive substances dangerous. When radiation is emitted, something changes in the composition of the atoms of that radioactive substance. Ultimately, a new atom arises, which cannot emit any more radiation and is therefore no longer radioactive. The radioactive substance has then decayed.
Types of radiation
Radioactive substances emit different sorts of radiation: alpha, beta, and gamma rays. Radiation can be blocked by shielding. Alpha rays have very little penetrating power. This radiation does not go any further than 5 to 6 cm into the air, and can be completely blocked by a piece of paper. Beta rays have a little more penetrating power, but can be blocked, for instance, by a 1 cm layer of water. Gamma rays and X-rays have strong penetrating power. To block this radiation, a 1.3m thick concrete wall may sometimes be necessary.
Background radiation
Natural radiation or background radiation is radiation emitted by naturally radioactive substances. These substances can be found closeby in the earth (e.g. uranium) or in the concrete we use to construct buildings. Natural sources of radiation are even found in our own bodies. Some natural radiation comes from far away, e.g. from the sun, other stars and supernovas. Background radiation is not equal everywhere on earth. Some places naturally have more radioactive substances in the ground and have a much higher level of background radiation than others. Height also plays an important role: the higher up you are, the less atmosphere there is to protect you against cosmic radiation. Finland, for instance, has lots of granite in its ground. Granite contains considerable uranium levels. On average, a person living in Finland is exposed to 7.5 mSv (milliSievert) in background radiation, while someone living in the Netherlands is exposed to an average amount of 1.6 mSv per year. The highest level of background radiation is measured in Ramsar, a city in the north of Iran. People there are exposed to a background dose of up to 260 mSv per year: over one hundred and sixty times more than in the Netherlands.

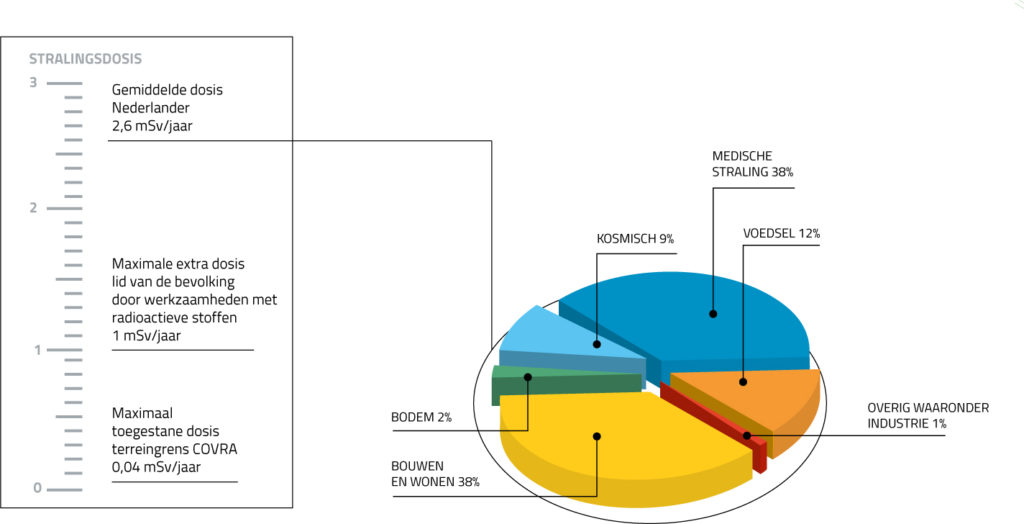
Radiation doses
The amount of radiation, or the radiation dose, is expressed in milliSievert (mSv). On average, people in the Netherlands are exposed to 1.6 milliSievert (mSv) of natural background radiation per year. In other countries this may be higher or lower. For most people in Europe, the dose is between 2 and 5 mSv per year. In Finland, the figure is considerably higher: 7.5 mSv per year. The differences are related to soil conditions; the soil in Finland, for instance, contains a lot of granite, while the distance to the sun also plays a role. Your job, any medical treatment and any examinations you may undergo can also influence the amount of radiation you are exposed to. A pilot, who is often closer to the sun, may receive an additional 5 mSv per year. Some famous DJs, like Hardwell, fly even more than pilots and are therefore exposed to even higher doses. If you take a transatlantic flight yourself, you will receive about 0.05 mSv. And a ski instructor as much as 8 mSv per season. At higher altitudes, the atmosphere above a person is thinner and therefore offers less-effective protection. An X-ray will expose you to approximately 0.1 mSv of radiation. On average, every Dutch person receives 1 mSv per year as a result of air travel and treatments or examinations with radioactive substances. Together with the natural background radiation, we receive approximately 2.6 mSv of radiation per person per year in the Netherlands.
Half-life
One property of radioactivity is that the radiation decreases with time. Nature takes care of this itself. The more time has lapsed, the weaker the radiation will be. This is due to the radioactive decay. The instable atoms are in search of a new balance. Once that balance has been found, the radioactive substance has stabilised and stops emitting radiation. How long this takes is expressed as half-life. This is the time required to keep losing half of the radioactivity. After two half-lives, radioactivity will have been reduced to half of half. In other words, a quarter of the initial value. Each radioactive material has its own fixed half-life. For one substance this may be just seconds, while others may need thousands of years. Some radioactive substances are therefore harmless as soon as they come into being. Other radioactive material needs to be stored for thousands of years before it stops emitting radiation.
The time it takes for radioactive substances to decay may range from a few days to billions of years.